Brain-gut microbiome interactions and functional bowel disorders
时间:2015年6月1日
地点:综合楼14楼会议室
主讲人:黄雅铃
内容简介:
肠易激综合征(IBS)是一组持续或间歇发作,以腹痛、腹胀、排便习惯和(或)大便性状改变为临床表现,而缺乏胃肠道结构和生化异常的肠道功能紊乱性疾病。肠道与神经系统相互作用的改变在IBS的发病机制起着重要的作用。大量的临床数据表明,肠道微生物可以调控肠与神经系统的相互作用。有研究通过描述IBS病人肠道菌群的改变和使用益生元、益生菌和抗生素后主观症状的改变来确定肠道生态失调在IBS症状发展中的一个小而不明确的作用。根据现有研究还有待明确的是IBS症状的发生是否与从肠道到微生物或者主要破坏的微生物的脑信号的改变有关,以及以上这些改变在IBS症状发展中是否参与改变大脑与肠道的相互作用。因此,在有很好表型的患者的大样本研究中描述患者的肠道菌群的改变是必要的,且要进一步找出肠代谢产物与肠-脑相互作用的具体的异常的关系。
作者:Emeran A. Mayer是加州大学大卫格兰医学院的一名教授,他在消化系统和神经系统如何影响健康和疾病的研究中拥有30多年的研究经验。近几年,他的研究集中在脑-肠相互作用领域,特别是肠道菌群对大脑结构和功能的影响,以及相关的行为和食物成瘾对肥胖的作用。
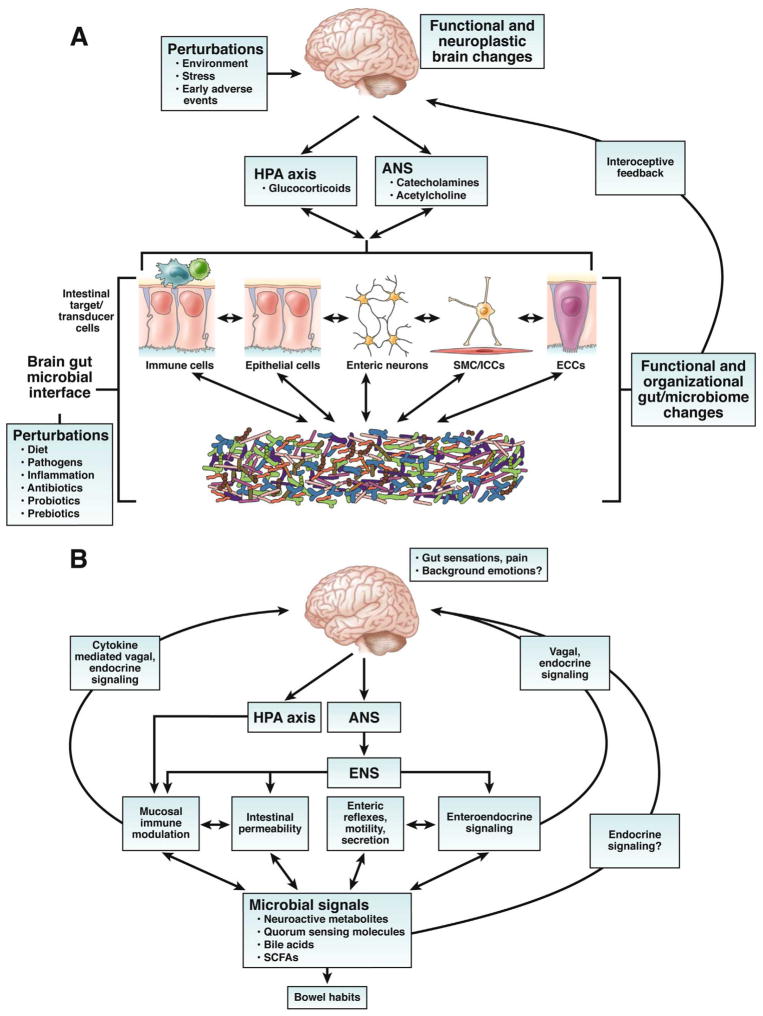
A. Key components of brain gut microbial axis. A network of specialized target/transducer cells in the gut wall functions as an interface between the organism and the gut lumen. In response to external and bodily demands, the brain modulates individual cells (ECC , enterochromaffin cells; SMC , smooth muscle cells; ICC , interstitial cells of Cajal) within this network via the branches of the autonomic nervous system (ANS) (sympathetic and parasympathetic/vagal efferents) and the hypothalamic pituitary adrenal (HPA) axis. Such modulation can be transient (e.g. in response to transient perturbations) or longlasting (in response to chronically altered brain output). The microbiota are in constant bidirectional communication with this interface via multiple signaling pathways, and this communication is modulated in response to perturbations of the microbiota, or the brain. The integrated output of the brain gut microbial interface is transmitted to the brain via multiple afferent signaling pathways, including endocrine and neurocrine (vagal, spinal afferents) pathways. While acute alterations in this interoceptive feedback result in transient functional brain changes, chronic alterations are associated with neuroplastic brain changes.
B. Functional and symptom-related consequences of brain gut microbial interactions. Several intestinal processes with possible relevance for IBS symptoms can be modulated both the brain (via the ANS, including its enteric nervous system [ENS] branch) and by signals from the microbiota. Microbiota generated molecules can signal to the brain indicrectly via activation of vagal (and possibly spinal) afferent nerve pathways, by microbiota stimulated cytokine and neurotransmitter release from immune or enteroendocrine cells, or such signals may reach the brain via an endocrine route. Microbiota gut brain signaling may contribute to the generation of abdominal pain and discomfort, while microbiota mediated modulation of enteric reflexes is likely to play a role in the pathophysiology of altered bowel habits.

